Pivotal Multicenter Trial of the Boomerang® Catheter for Percutaneous Deep Vein Arterialization (pDVA)
You Play a Vital Role in Preventing Amputations.
As a healthcare professional, you recognize the need for an effective treatment option for patients with Chronic Limb-Threatening Ischemia (CLTI), the most advanced form of peripheral arterial disease (PAD). When blood flow to the lower leg and foot is significantly limited or cut off, the result is severe leg pain and wounds that won’t heal which may ultimately require amputation.
Aveera Medical has developed a unique device, the Boomerang® Catheter, that may help restore blood flow to the foot, by a procedure known as deep venous arterialization (DVA).
The Boomerang Trial is studying whether this investigational, minimally invasive medical procedure can prevent lower leg amputation and promote wound healing.
Patients may qualify for the study if they:
√ Suffer from Chronic Limb-Threatening Ischemia
√ Experience severe leg pain, even when at rest
√ Have non-healing foot wounds
√ Have received a recommendation to consider amputation of the foot or leg
√ Conventional endovascular or surgical limb salvage treatments have failed

Participating Sites
Click on site to contact a trial coordinator
Site Name
Site Location
Physician
The Aveera Boomerang Catheter
Uses time, pressure, and heat (Direct Current, not RF) to create an immediate and fused anastomosis between the vein and artery without the use of a stent
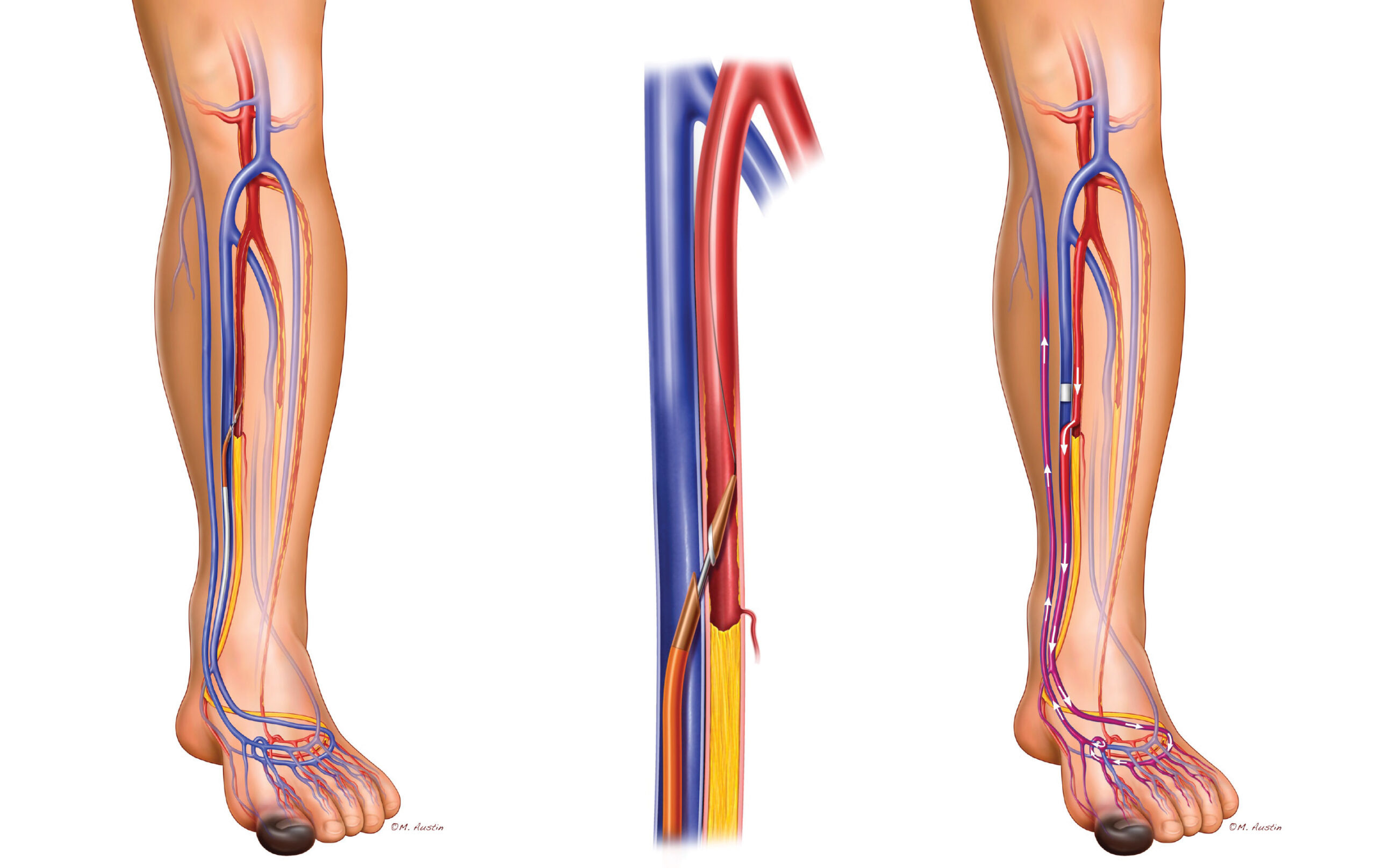
√ Enables endovascular arteriovenous anastomosis creation
√ Requires no stents or stent grafts
Contact Us
Caution – INVESTIGATIONAL DEVICE, LIMITED BY FEDERAL (OR UNITED STATES) LAW TO INVESTIGATIONAL USE. ClinicalTrials.gov ID: NCT06311773
© 2025 Aveera Medical, Inc. All rights reserved.

